Can’t-Miss Takeaways Of Tips About How To Tell If A Substance Is Ionic
So you are only allowed one test?
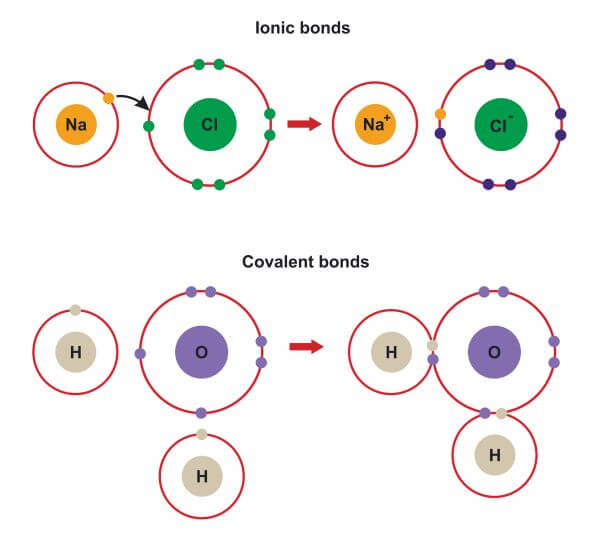
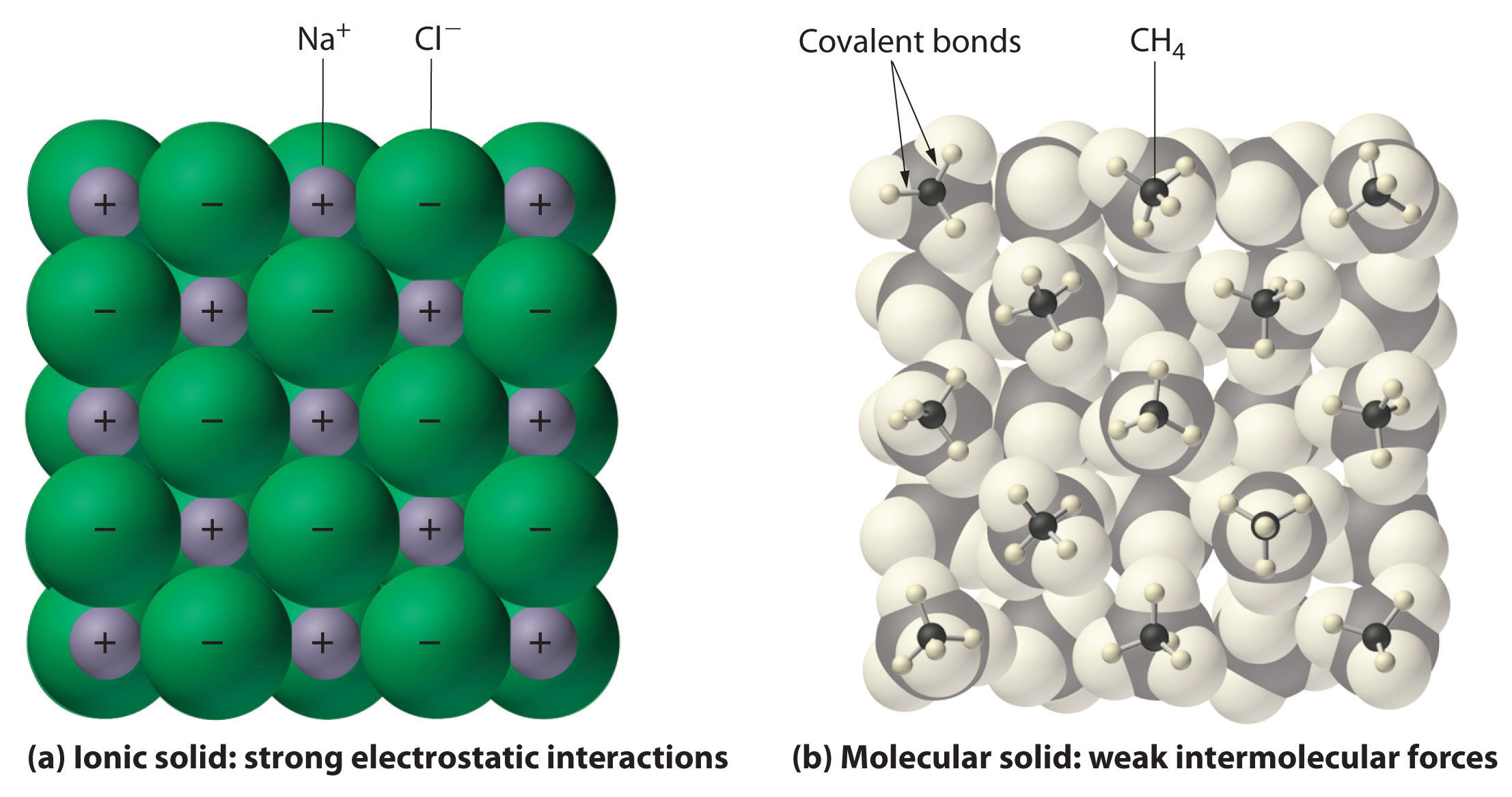
How to tell if a substance is ionic. Result answer link. So, for example, at the end of this experiment, if the sample remained unmelted, dissolved in water and conducted. Result in ionic bonding, atoms transfer electrons to each other.
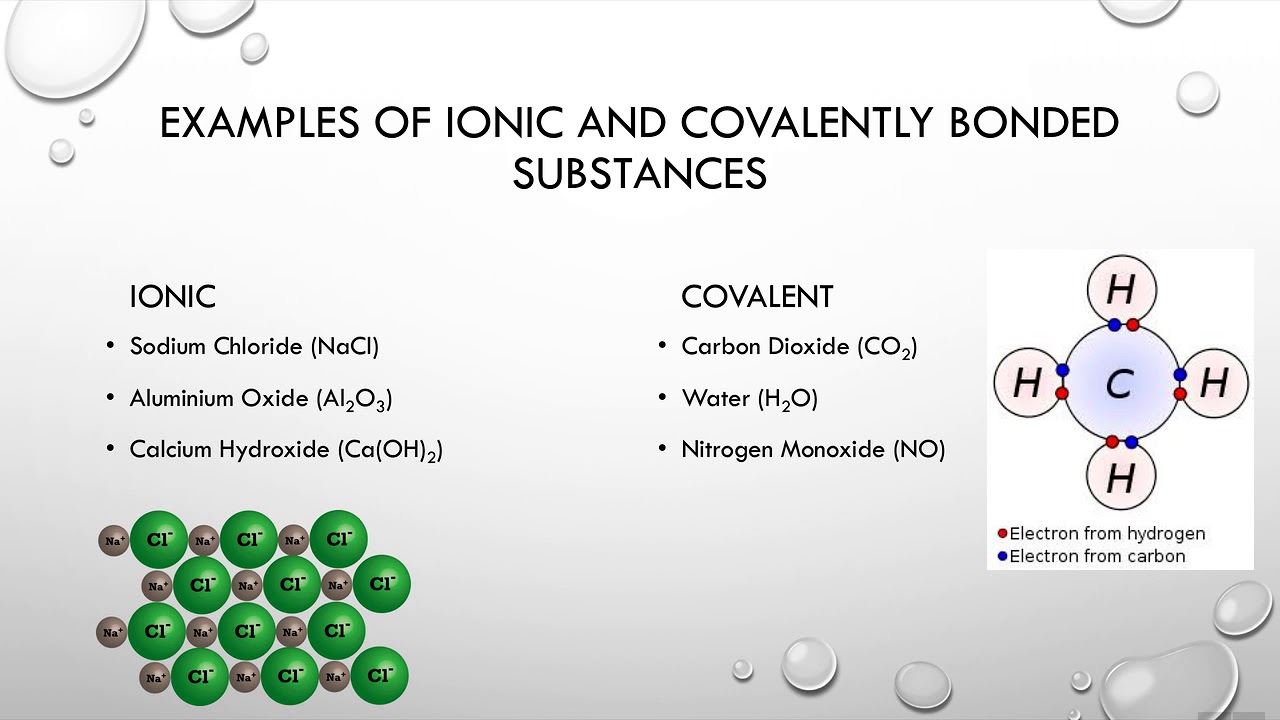
What is an ionic compound? Result as it turns out, there are very few strong acids, which are given in table 14.7.1. Predict the type of compound formed from elements based on their location within the periodic table.
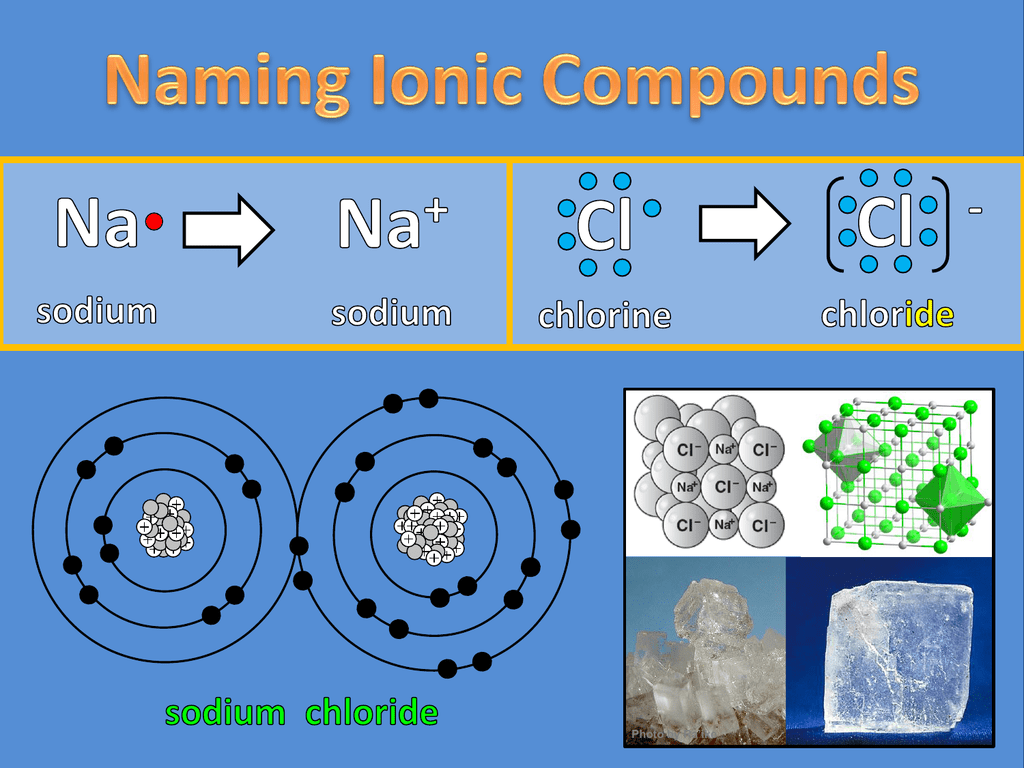
Result identify ions present in ionic compounds. The compounds formation takes place with the two elements. Result how do you identify an ionic compound?
The periodic table can help us recognize many of the compounds that. Table \(\pageindex{1}\) comparison of ionic and. Result complete step by step solution:
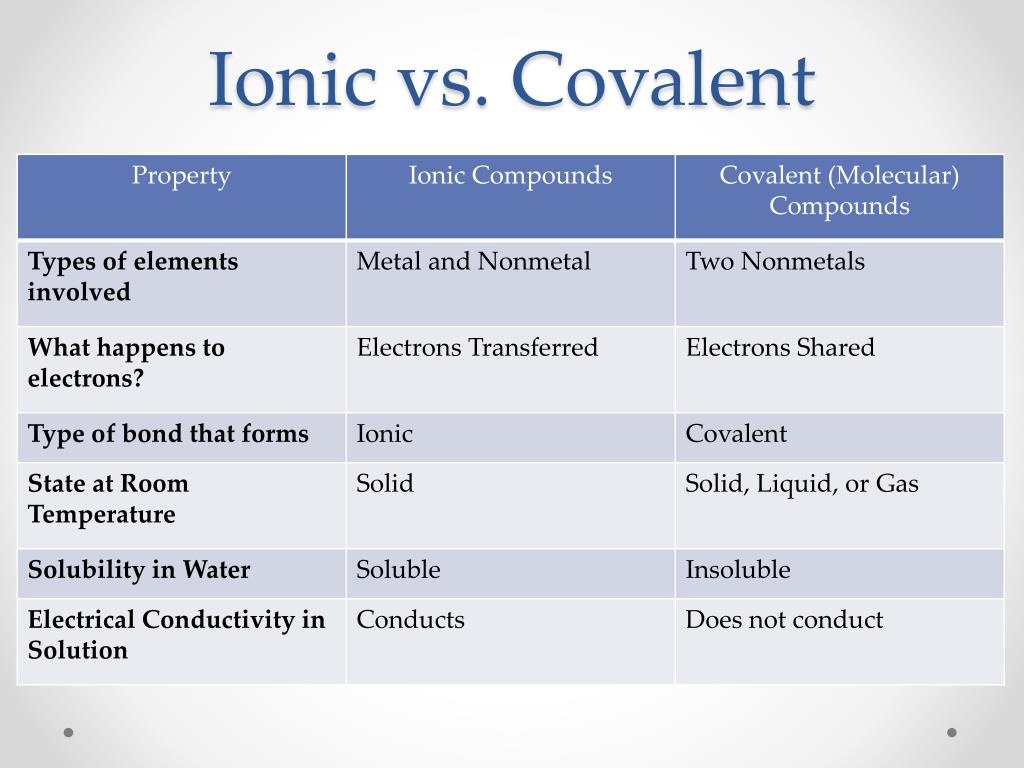
Covalent compounds are generally made up of what kind of. We can determine whether the substance. Result you can predict an ionic bond will form when two atoms have different electronegativity values and detect an ionic compound by its properties,.
To identify an ionic compound: Result how do you determine if a substance is an ionic compound? Result if the difference between the electronegativities is large, the more electronegative atom will take the bonding electrons completely away from the.
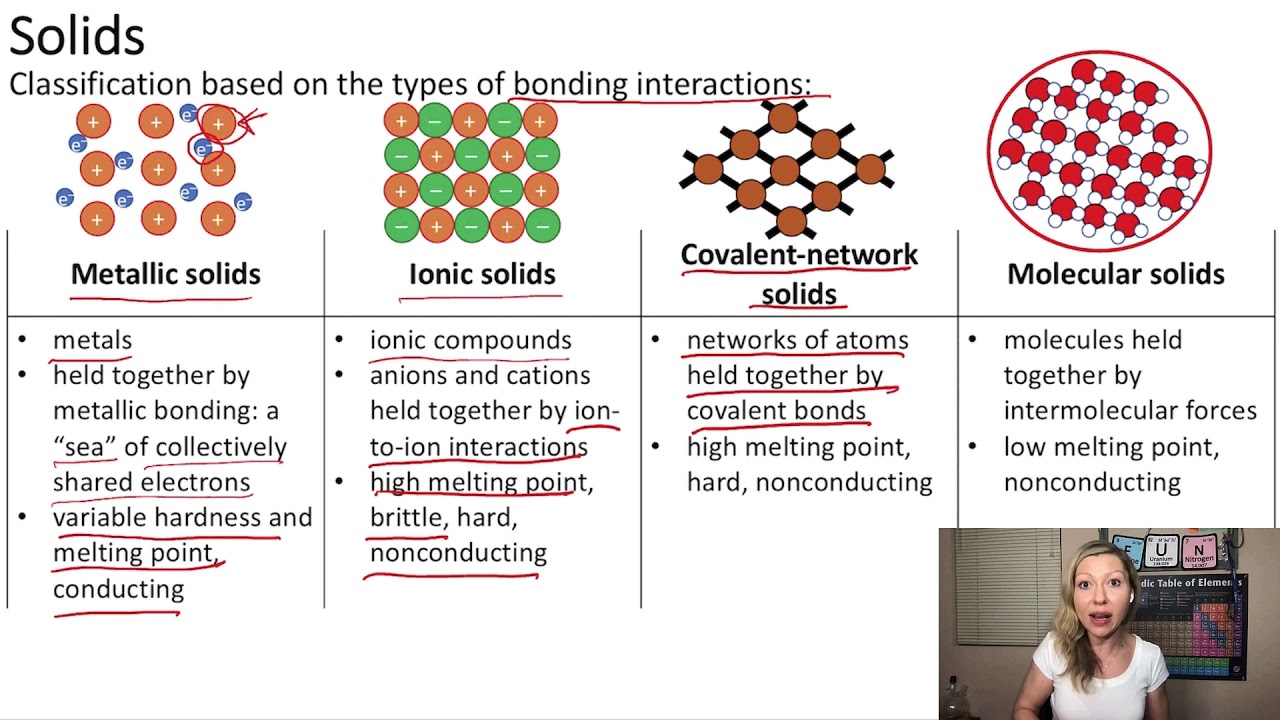
It may be 1% ionized. The ionic compounds have different properties than that of covalent or metallic compounds. There is a simple and easy way to identify ionic versus covalent compounds.
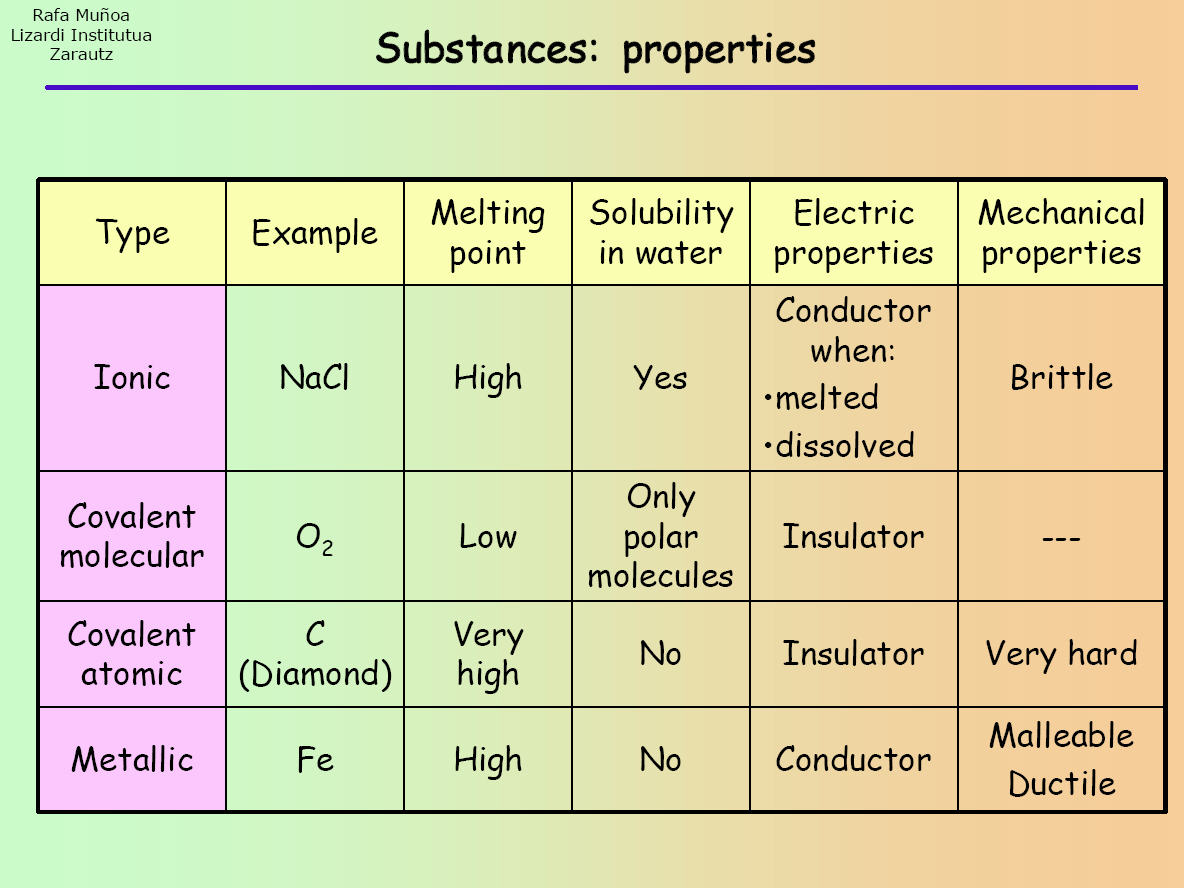
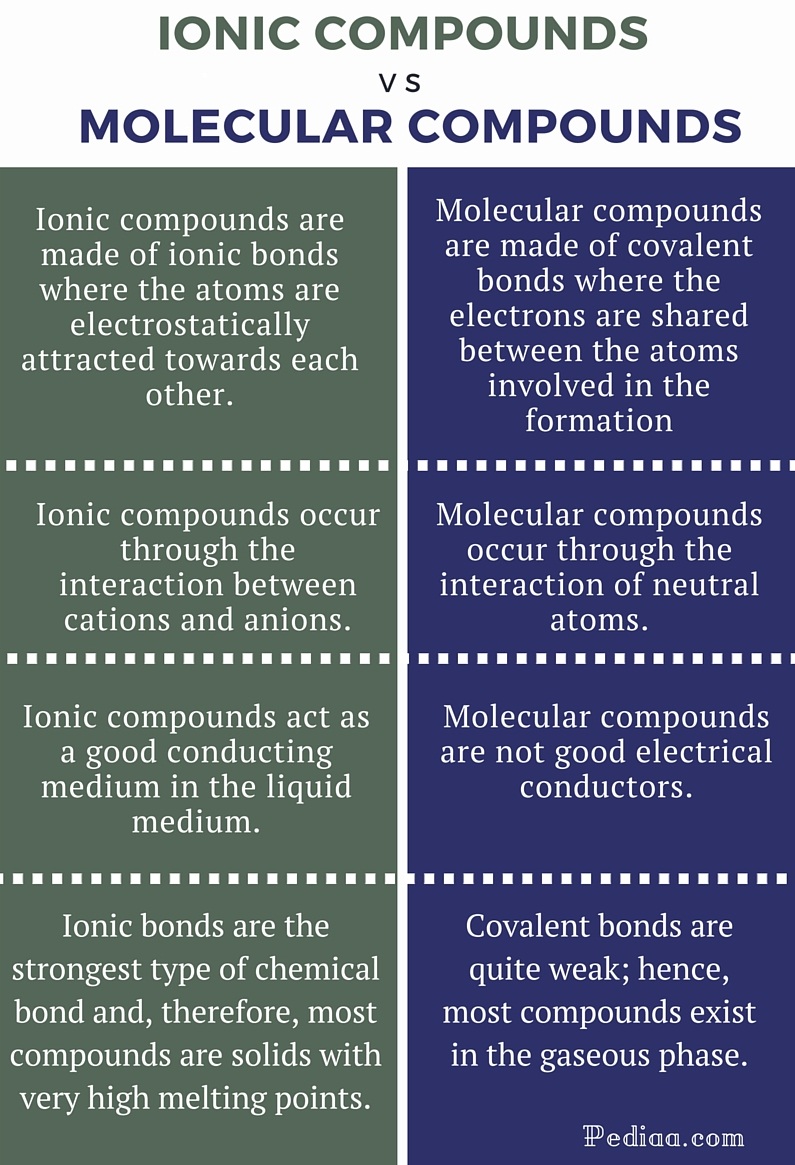
Ionic compounds are pure substances consisting of. Result the table below summarizes some of the differences between ionic and molecular compounds. Result how to identify an ionic vs covalent compound?
Result define ionic and molecular (covalent) compounds. Try melting the substance and see if it conducts electricity in the liquid state (yes=ionic). Result a bond is ionic if the electronegativity difference between the atoms is great enough that one atom could pull an electron completely away from the other one.
See the study guide on the three states of matter. If an acid is not listed here, it is a weak acid. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature.