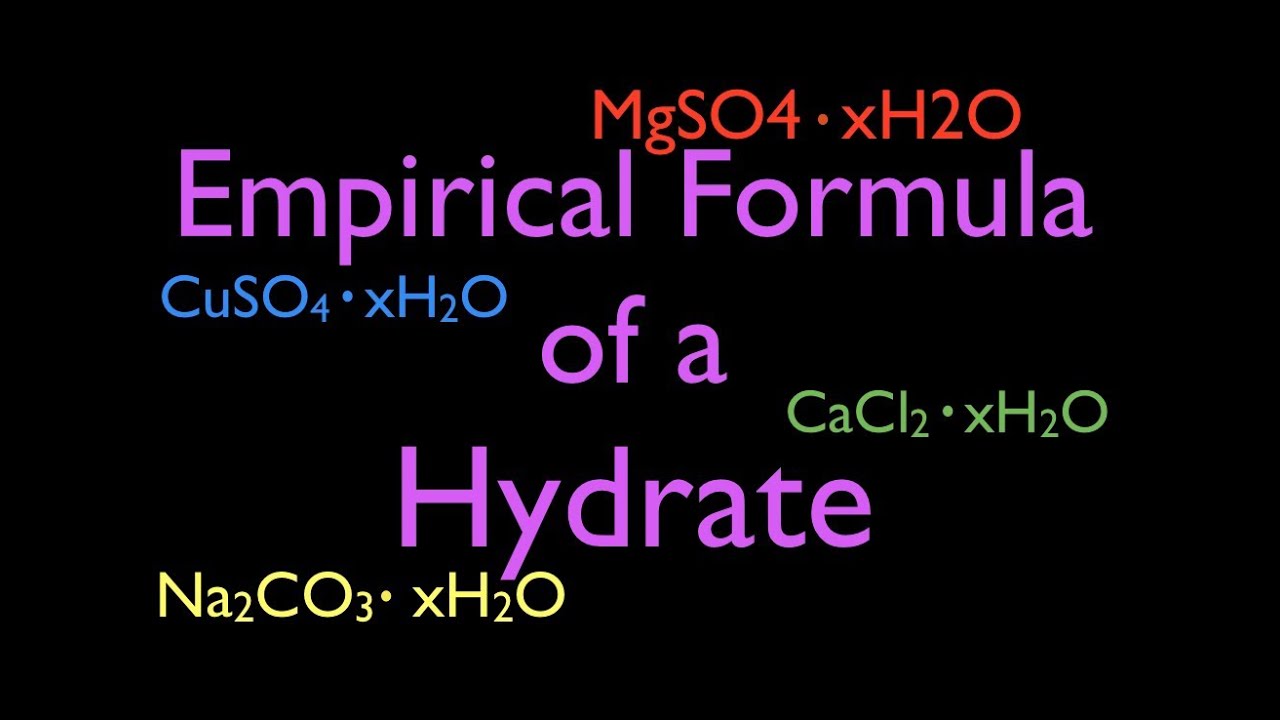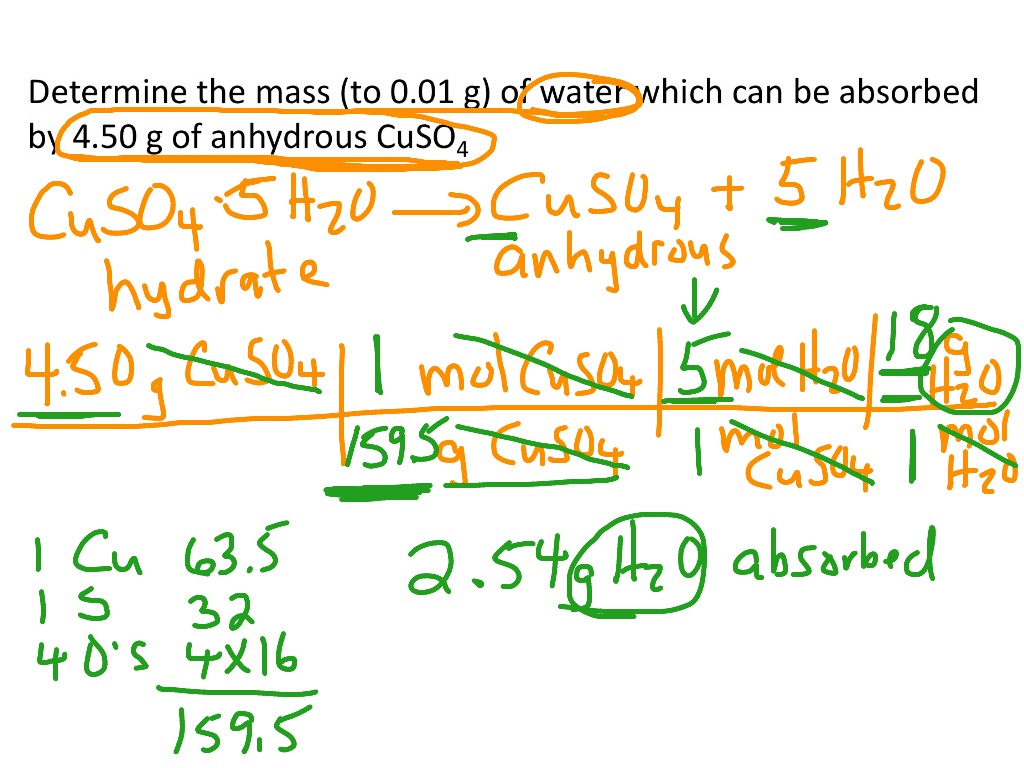Best Tips About How To Write A Hydrate Formula

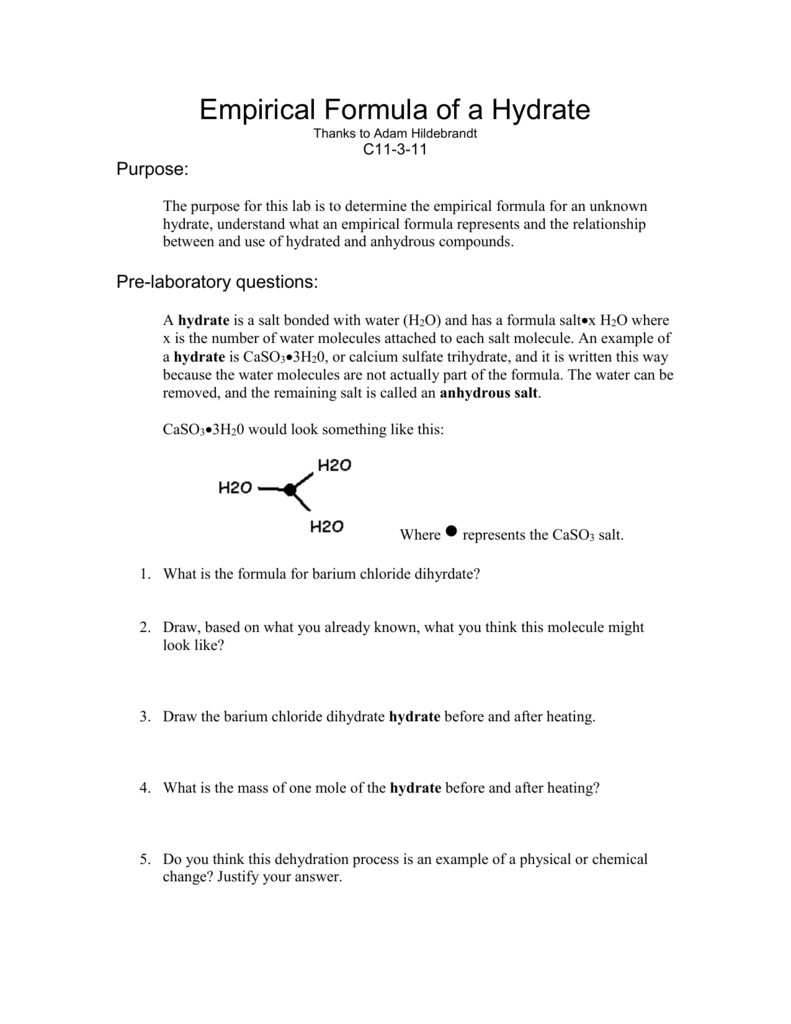
A hydrate contains a definite number of water molecules bound to each ionic compound (also called the anhydrous salt).
How to write a hydrate formula. The number of moles of water. In this guide, we give a complete explanation of hydrates, including the hydrate item, the threes different types of hydrates, the regels thou need to known to name hydrates and. Thoroughly wipe a crucible and cover with a clean cloth towel to remove dirt and other particulate matter.
Determination of the formula of a hydrate. In order to determine the formula of the hydrate, [\(\text{anhydrous solid}\ce{*}x\ce{h2o}\)], the number of moles of water per mole of anhydrous solid (\(x\)). Start with the most complicated formula.
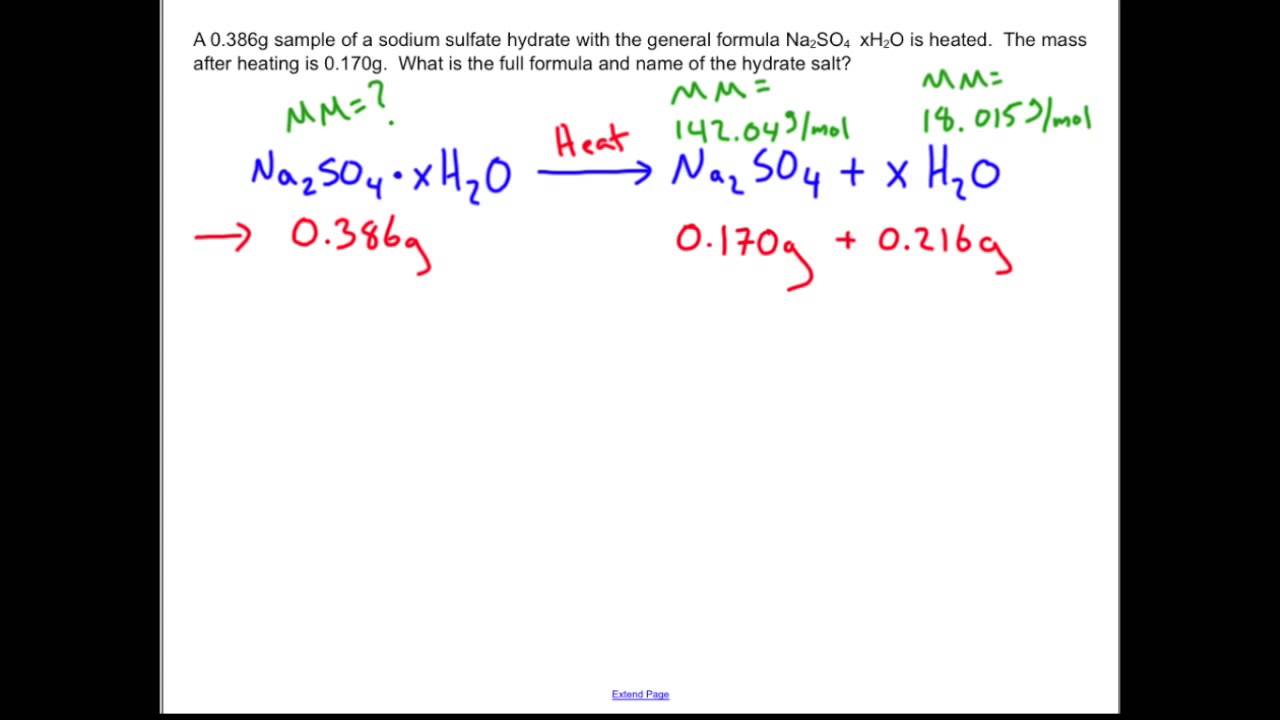
Each student will work. Mass of hydrate = mass of h2o lost + mass of anhydrous salt after heating. Hydrates are inorganic salts which.
1 ba (oh)₂·8h₂o + hno₃ → ba (no₃)₂ +. The formula for water is set apart at the end of the formula with a dot (•),. In this guide, we give a complete explanation of hydrates, including the hydrate definition, the three dissimilar forms of hydrates, the rules thee need to know to.
Acid and base catalyzed formation of hydrates and hemiacetals. Modified 1 year, 7 months ago. In this guide, we give a complete explanation of hydrates, including this hydrate definition, the three different types of hydrates, an rules you need to know to designate hydrates.
Hydrates are inorganic salts containing water molecules combined in a definite ratio as an integral part of the crystal [1] that are either bound to a metal center. Determine the percent water of hydration in a hydrate sample. The law of definite proportions or constant composition states that the elements in a pure compound.
The purpose of this experiment is to determine the empirical formula of a hydrate. In the formula mgso 4 nh 2 o, what does the n before the h 2 o stand for? The number of moles of hydrogen.
Determine the mass of the crucible (and cover). How do you write fractional hydrates in words? In contrast, copper sulfate usually forms a blue solid that contains five waters of hydration per formula unit, with the empirical formula cuso 4 ·5h 2 o.
What is the enthalpy of hydration? The number of moles of hydrate. Hydrates are substances that include water in their formula.
The formula of the hydrate is represented. Enthalpy of hydration, \(\delta h_{hyd}\), of an ion is the amount of heat released when a mole of the ion dissolves in a. Kaygeesthetics on february 28, 2024: